Separation of Mixture Containing Maximum Boiling Azeotrope with Extractive Heterogeneous-Azeotropic Distillation
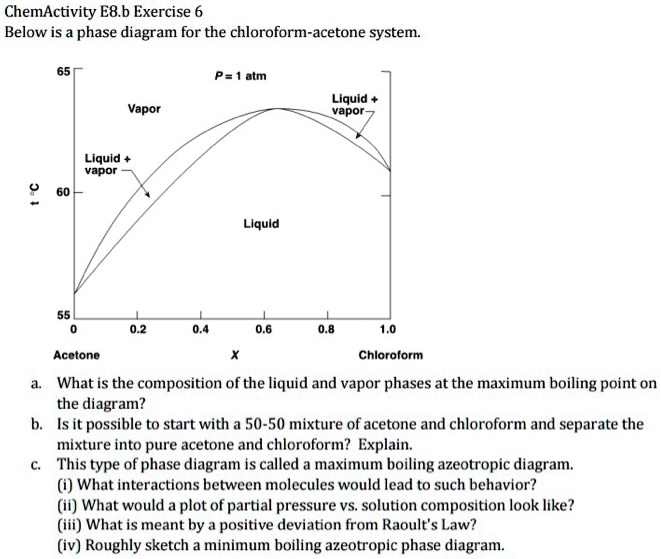
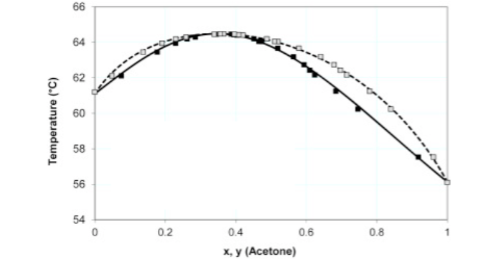
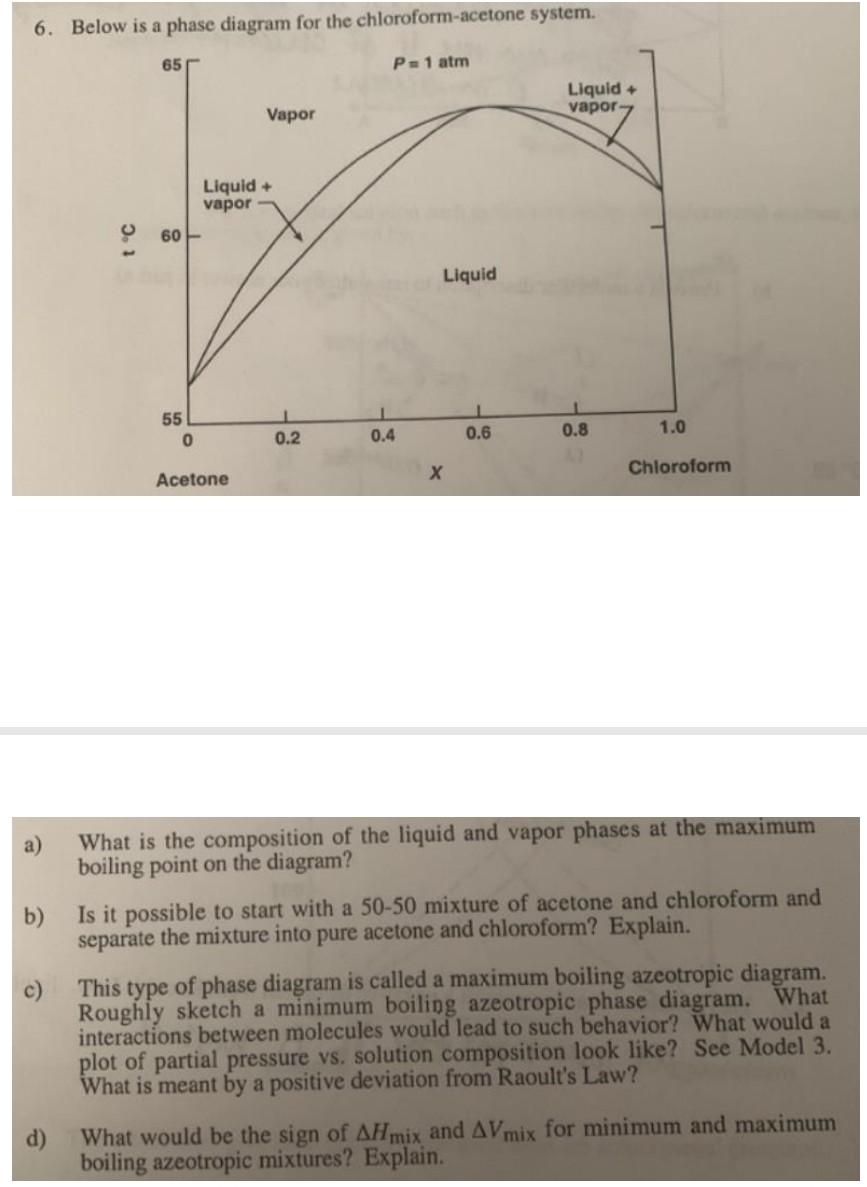
SOLVED: ChemActivity E8.b Exercise 6: Phase Diagram for the Chloroform- Acetone System Below is the phase diagram for the chloroform-acetone system: P = atm Liquid vapor Vapor Liquid vapor Liquid Acetone Chloroform What

Control of the Maximum-Boiling Acetone/Chloroform Azeotropic Distillation System | Industrial & Engineering Chemistry Research
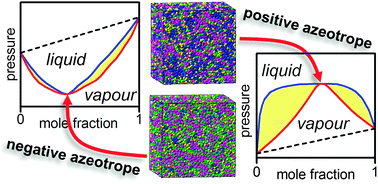
Microstructures of negative and positive azeotropes - Physical Chemistry Chemical Physics (RSC Publishing)

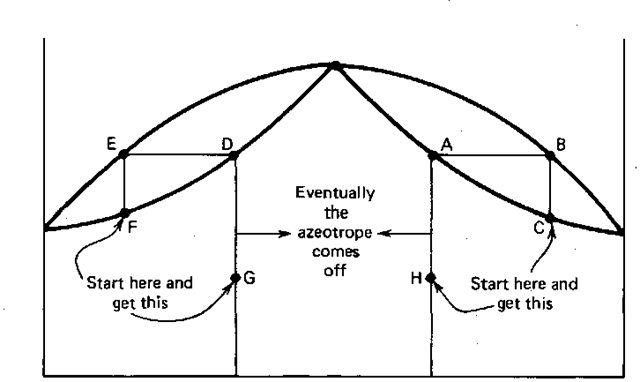
SciELO - Brasil - THERMODYNAMIC TOPOLOGICAL ANALYSIS OF EXTRACTIVE DISTILLATION OF MAXIMUM BOILING AZEOTROPES THERMODYNAMIC TOPOLOGICAL ANALYSIS OF EXTRACTIVE DISTILLATION OF MAXIMUM BOILING AZEOTROPES
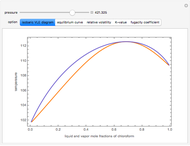
High Pressure Vapor-Liquid Equilibrium Data of a Binary Mixture of Chloroform and Acetone - Wolfram Demonstrations Project

Heterogeneous Azeotropic Extractive Coupled Heat Pump Distillation for Triethylamine‐Dichloromethane‐Acetone‐Water - Yang - 2023 - Chemical Engineering & Technology - Wiley Online Library

Applied Sciences | Free Full-Text | Effect of Ionic Liquids on the Isobaric Vapor-Liquid Equilibrium Behavior of Acetone-Chloroform

Figure 3 from Separation of Acetone-chloroform maximum boiling azeotrope using Dimethyl sulfoxide | Semantic Scholar
Steady State Design for the Separation of Acetone-Chloroform Maximum Boiling Azeotrope Using Three Different Solvents

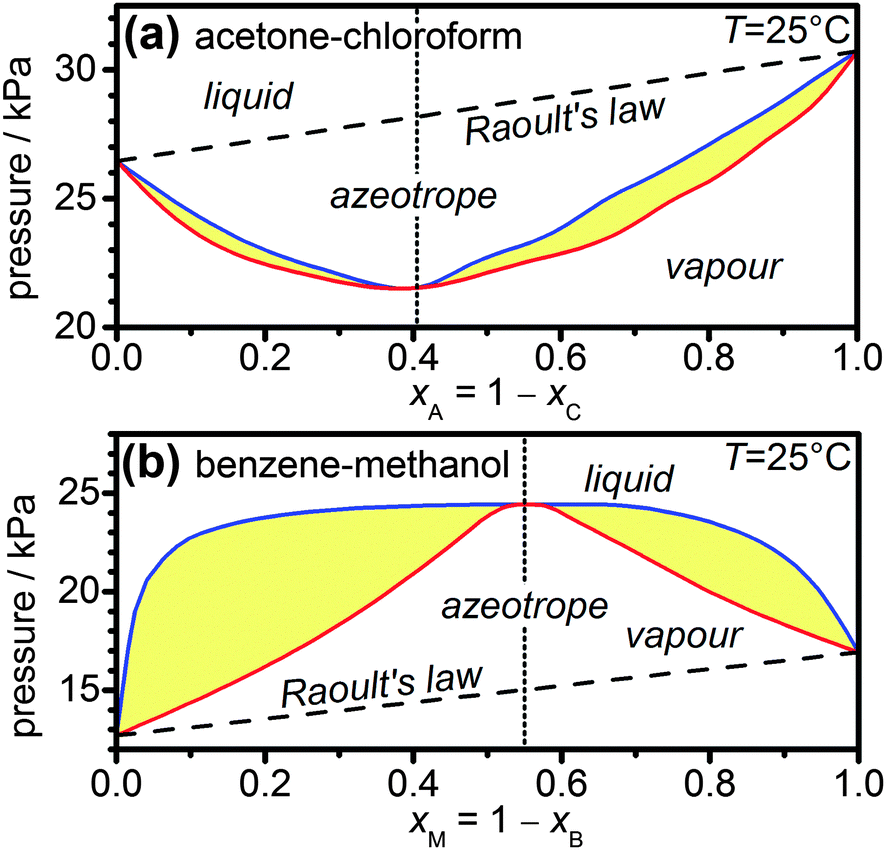
Vapour pressures of pure acetone and chloroform 328 K are 741.8 mm Hg and 632.8 mm Hg respectively. Assuming that they form ideal solution over the entire range of composition, plot ptotal,pchloroform,

Control of the Maximum-Boiling Acetone/Chloroform Azeotropic Distillation System | Industrial & Engineering Chemistry Research
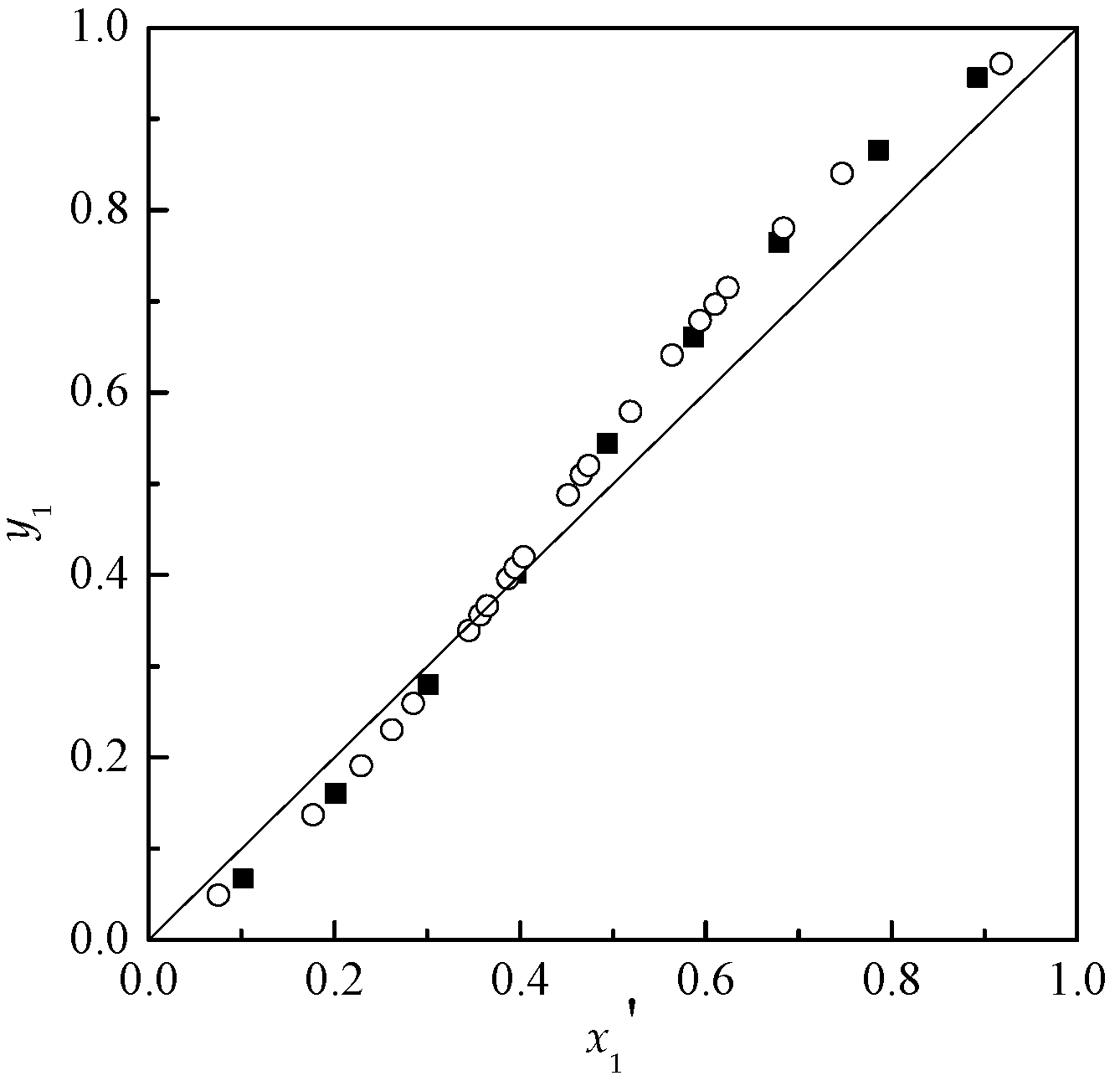
Applied Sciences | Free Full-Text | Effect of Ionic Liquids on the Isobaric Vapor-Liquid Equilibrium Behavior of Acetone-Chloroform