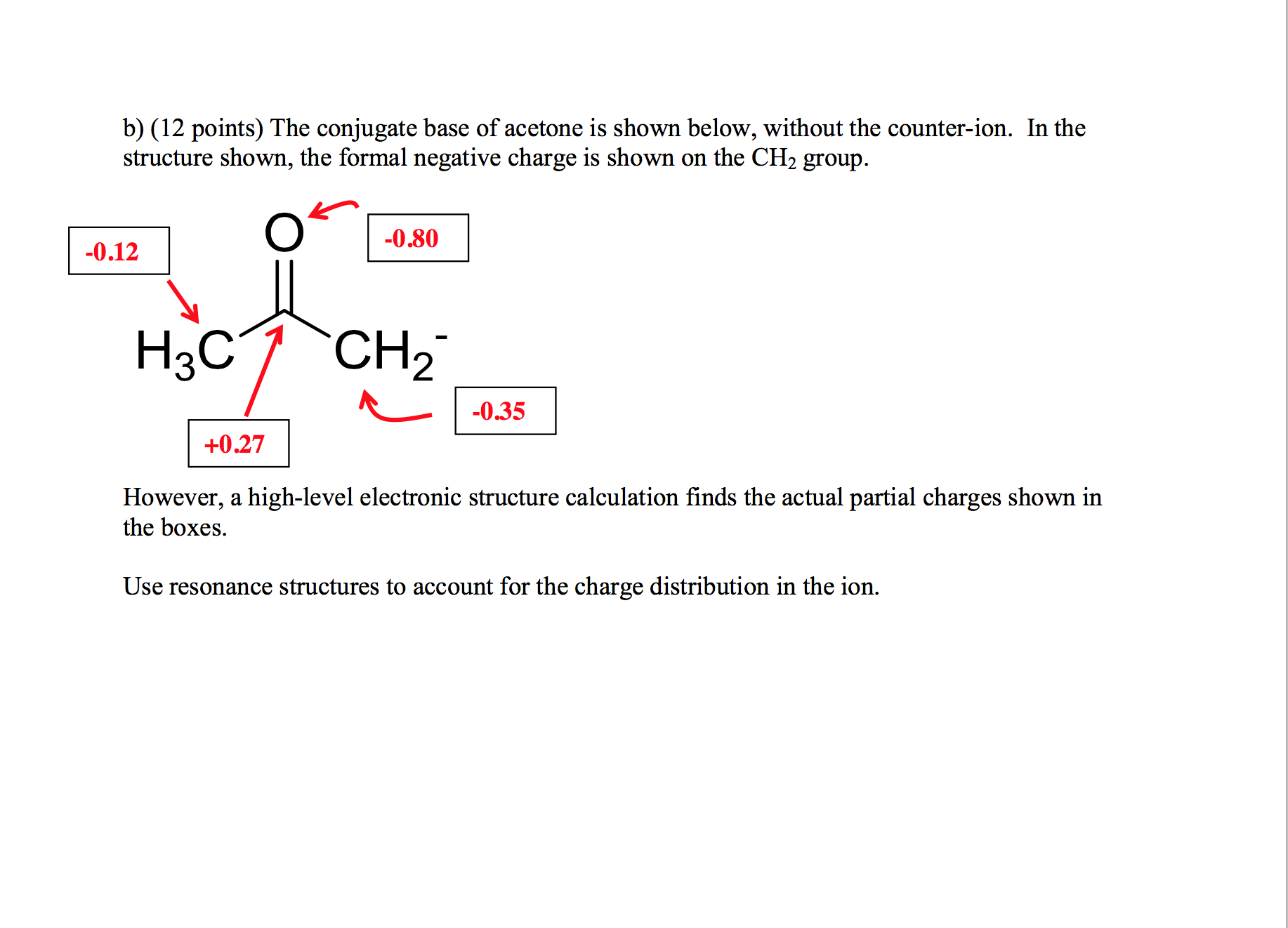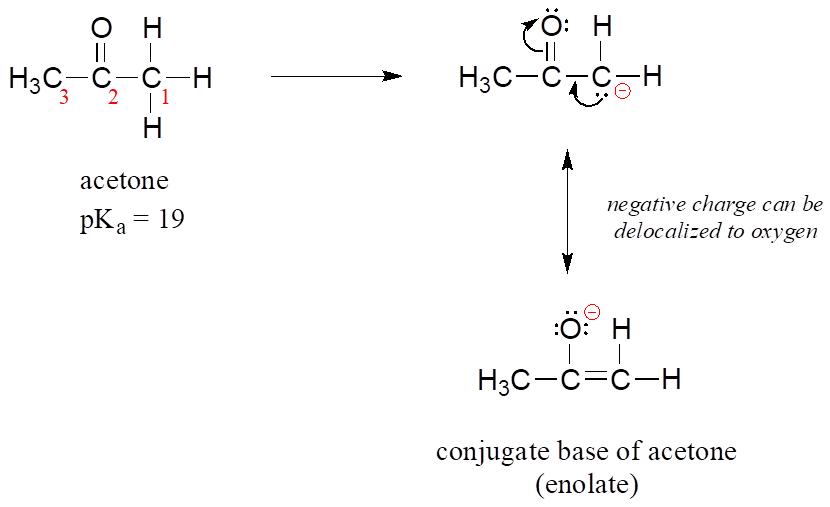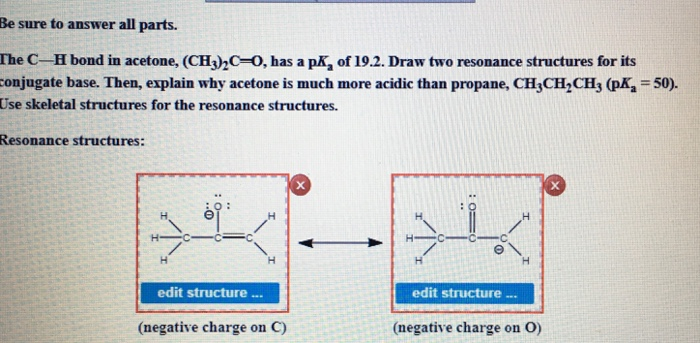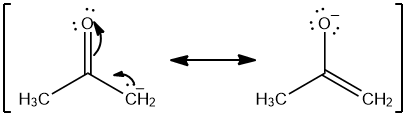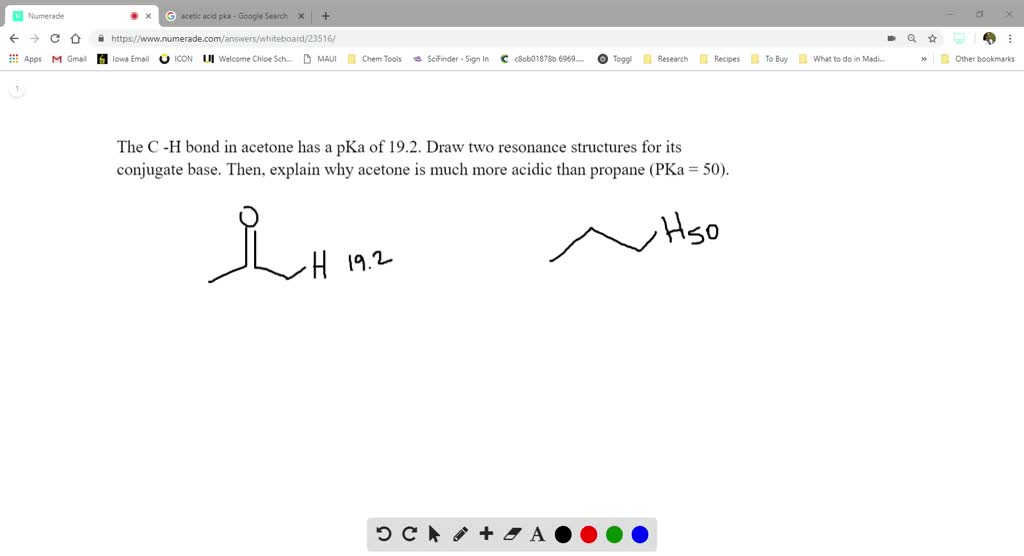
SOLVED:The C-H bond in acetone, (CH3)2C = 0, has a pKa of 19.2. Draw two resonance structures for its conjugate base. Then, explain why acetone is much more acidic than propane, CH3CH2CH3 (

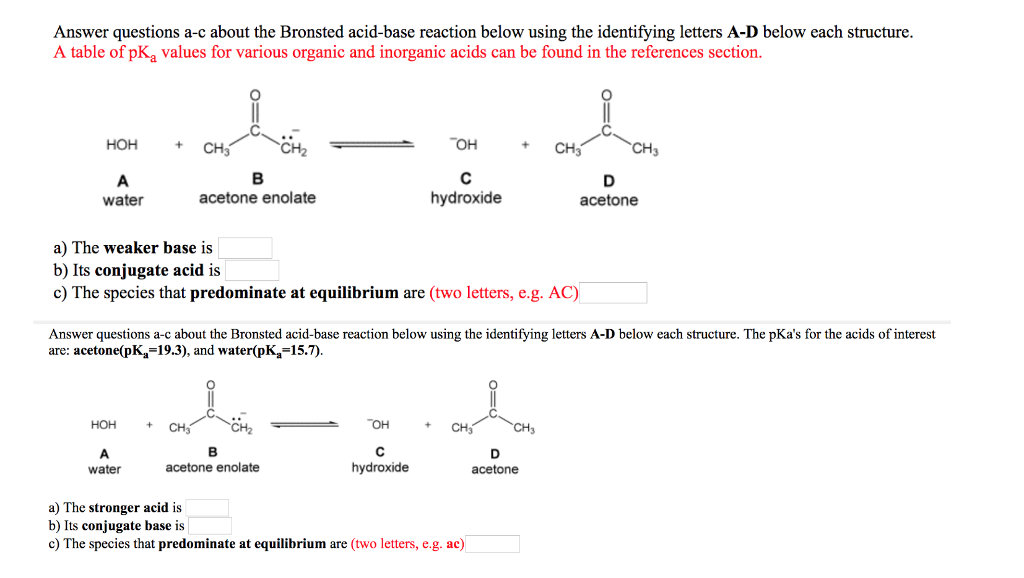
OneClass: Answer questions a-c about the Bronsted acid-base reaction below using the identifying lett...

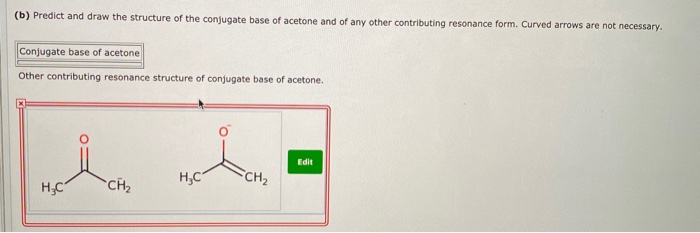
OneClass: Draw the structure of the conjugate acid of acetone (CH3COCH3) Draw the structure of the co...

2,4-Pentanedione is a considerably stronger acid than is acetone. Write a structural formula for the conjugate base of each acid and account for the greater stability of the conjugate base from 2,4-pentanedione.
2.7 Acids and Bases: The Brønsted-Lowry Definition Acids and Bases: The Brønsted-Lowry Definition Acids and Bases: The Brønst

OneClass: Answer questions a-c about the Bronsted acid-base reaction below using the identifying lett...

organic chemistry - Acidity order of acetone, methyl acetate and N,N-dimethylacetamide - Chemistry Stack Exchange

A New Class of Chiral Pyrrolidine−Pyridine Conjugate Base Catalysts for Use in Asymmetric Michael Addition Reactions | Journal of the American Chemical Society

The C-H bond in acetone, (CH3_)_2C=0, has a pKa of 19.2. Draw two resonance structures for its conjugate base. | Homework.Study.com